AN EVIDENCE-BASED APPROACH TO UNDERSTANDING DEFORMATIONAL CRANIAL FLATTENING
Introduction
Based on a mounting body of evidence that supine positioning of infants during sleep may reduce the incidence of sudden infant death syndrome, the American Academy of Pediatrics initiated the Back to Sleep Campaign in 1992. The recommendation was widely followed in the West and is credited with a 40% reduction in the incidence of sudden infant death syndrome in the United States. One of the unforeseen consequences of the campaign was an exponential rise of occipital flattening, termed plagiocephaly (asymmetrical) or brachycephaly (symmetrical). Similar cranial shape changes had been observed in prone-slept infants, but with a much lower prevalence and severity of flattening. Current estimates suggest that “abnormal” deformational cranial flattening occurs in 18% to 19.7% in healthy infants, but this depends largely on how one defines “abnormal”, a topic of great debate even among experts in the field.
Terminology- Deformational Plagiocephaly, Brachycephaly, Scaphocephaly
Typical right posterior deformational plagiocephaly; note anterior shifting of right forehead and ear.
Deformational cranial flattening can take many forms depending on which part of the cranium contacts the resting surface. Most clinicians incorrectly refer to any type of cranial flattening as plagiocephaly, but the term is correctly applied only to asymmetrical flattening. Deformational plagiocephaly occurs in infants who consistently turn their head to one side, or appear to have a “favorite head position”. Although some people incorrectly ascribe this to the environment or some infant “preference”, it is a mechanical limitation that is almost always a result of an imbalance in the neck muscles termed congenital muscular torticollis (CMT). Consistent contact pressure in one area of the cranium effectively restricts growth in that region and, with continued growth, the cranial shape becomes progressively asymmetric. While the shape has been compared to a parallelogram, the frontal asymmetry is always less than that in the occiput and the shape is really more trapezoidal. Asymmetric growth of the cranium is often accompanied by compensatory facial asymmetry, specifically an anterior shift of the ipsilateral forehead, ear, and cheek on the same side as the posterior flattening. Asymmetric opening of the palpebral fissures can be observed as a consequence of the sagittal displacement of the ipsilateral zygoma and tensioning of the canthal tendons. The vertical palpebral asymmetry is often confused with contralateral eyelid ptosis.
Deformational plagiocephaly can be confused with similar appearing forms of craniosynostosis: unilateral coronal synostosis (UCS) and lambdoidal synostosis. First, it is important to recognize that these conditions are very rare compared to deformational flattening. Unilateral coronal synostosis, or premature closure of one coronal suture, causes severe frontal flattening of the forehead on the same side as the more open eye. Also, the orbital rim on the affected side is often posterior to the anterior globe. Patients with UCS often have flattening in the occipital area on the fused side as well, but this is not deformational; instead it is the result of an angular deformity in the cranial base. The nasal root and midface are also angulated (not seen in DP) and there is anterior displacement of the ear on the same side as the forehead flattening (the opposite of DP).
Lambdoidal synostosis, or synostotic posterior plagiocephaly, can be difficult to differentiate from DP. In fact, diagnostic confusion at several large academic institutions led to a number of patients with DP undergoing unnecessary operative intervention. The practice of operatively managing DP subsided after a large public investigation clarified the clinical and prognostic distinction between the two entities. To the trained eye, the appearance of DP is easily distinguished from lambdoidal synostosis- there is a wind-swept appearance to the head due to asymmetric cranial height on the flattened side (opposite of DP, which is rarely as obvious) and lambdoidal synostosis has mastoid bossing on the fused side, a finding uncharacteristic in DP. Lastly, patients with lambdoidal synostosis have little to no forehead or cheek asymmetry as since the cranial base angulation is almost entirely in the posterior fossa.
Posterior views of infant with right lambdoid craniosynostosis (above) and left posterior deformational plagiocephaly (right). The height of the cranial vertex is lower on the affected side for the craniosynostosis patient but higher in the patient with deformational changes. The vertical position of the ears (level) is only significantly altered in the patient with craniosynostosis.
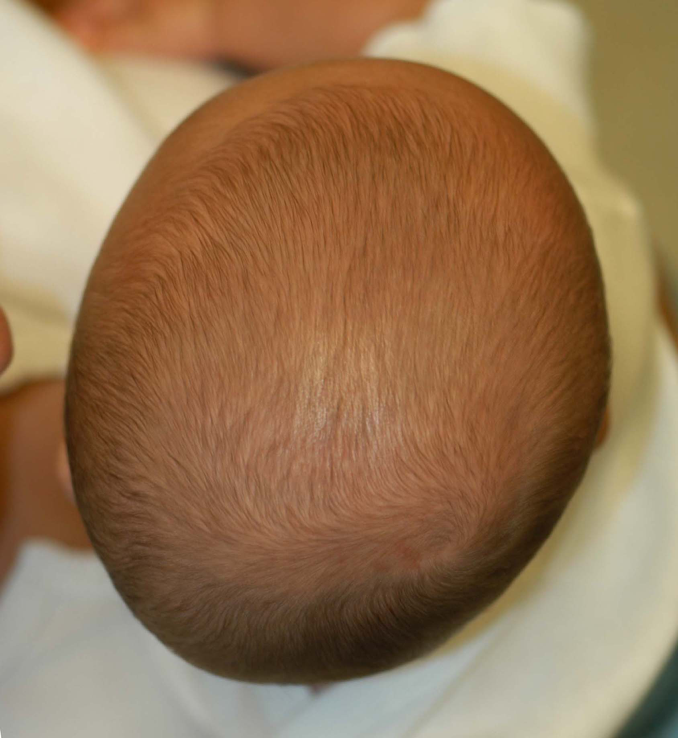
Brachycephaly implies a symmetrical occipital flattening and compensatory biparietal widening. Infants with deformational brachycephaly (DB) have little or no rounding on the back of the head and appear to have a disproportionately wide or big head viewed from the front. The posterior vertex may appear taller than the front (turricephaly), giving a sloped appearance to the head in profile. The ratio of cranial width to length, termed the cranial index or CI, is higher than normal. This figure is historically 0.75 to 0.80 in North America, although some observers suggest that the normal CI has risen to 0.8 to 0.85 as a result of back sleeping. Most children with DB also have some element of asymmetry and this combination is termed asymmetric brachycephaly, This the most common type of deformational cranial shape. Brachycephaly can also be seen in infants with craniosynostosis when both coronal sutures are fused. Synostotic brachycephaly is relatively rare and has features not seen in DB: severe forehead retrusion (superior orbital rim is behind the anterior surface of the globe- the eyes appear very prominent) and generalized, not regional, turricephaly.
Deformational brachycephaly; symmetrical flattening. There is no facial asymmetry.
Asymmetric deformational brachycephaly; asymmetry with a broad head.
Deformational scaphocephaly; this subtype, due to the very lateral area of flattening, has the most pronounced facial asymmetry.
Deformational scaphocephaly (DS) is a less common variant of plagiocephaly seen in infants who have extreme head rotation to one side or in premature infants who are positioned side-to-side in the intensive care units. Flattening develops on the side(s) of the head, with compensatory growth in the anterior and posterior cranium. These infants tend to develop a long, slender head that some refer to as a “toaster head”. There is often relatively pronounced facial asymmetry. This presentation can be confused with synostostotic scaphocephaly associated with sagittal craniosynostosis. Unlike DS, however, sagittal synostosis typically results in frontal bossing, bilateral occipital/parietal narrowing posterior to the anterior fontanelle, and decreased vertical height of the posterior cranium. Facial asymmetry is also rare in sagittal synostosis. Additionally, most infants with this type of craniosynostosis have a head circumferences in excess of the 90th percentile.
The Mechanism of Cranial Flattening: How does an infant’s head become flat?
Several hypotheses have been proposed to explain deformational calvarial flattening. It is often suggested that the infantile cranium is soft or malleable and that this predisposes the bony plates to deform when the head lies on a planar surface. This proposed mechanism is analogous to the type of shape distortion that occurs when a water balloon is placed on a table. This concept is easily dismissed by merely placing a newborn on a firm, flat surface; the head does not immediately deform. Furthermore, because all infants would conceptually have soft heads, one would expect every newborn to undergo cranial flattening if this were true. In reality, less than a quarter of infants positioned on their back develop visible cranial flattening, and the severity peaks around 4 months of age. In an attempt to reconcile these facts, some have proposed that susceptible infants may have an inherent problem with bone mineralization that makes them more susceptible. There is no evidence to support this hypothesis either.
Another common, but generally incorrect, theme is that cranial flattening is hereditary. It is not uncommon for the parents to suggest that some member of the extended family has a similar cranial shape. This is particularly true in families whose cultural traditions include back sleeping. Although ethnic variations in the growth of the cranial base are largely genetic in origin, growth of the neurocranium (calvaria) occurs passively in response to expansion of the brain and intracranial contents. Because the human cerebrum is not naturally flat or asymmetric, flattening can occur only when external force is exerted by a planar surface.
To better understand the mechanism of cranial deformation, it is instructive to look back in history. Intentional cranial deformation is the volitional alteration of normal head shape. This was practiced by many cultures including the ancient Peruvians, the North American Chinook Indians, and the French aristocracy. In most instances, cranial deformation was accomplished by applying a constant external force, usually exerted by a board or cloth wrap, to the growing head. Over time, the natural shape of the head was permanently altered in a predictable and, presumably, culturally desirable way. Although intentional cranial deformation is no longer practiced, some culturally based rearing practices can lead to unintentional changes in cranial shape. For example, swaddle boards (hard, flat infant resting surface) are used in some Asian cultures. Swaddling diminishes infant mobility and, when coupled with supine positioning on a hard, flat resting surface, this results in a high rate of occipital flattening. Not surprisingly, cranial flattening is more common and culturally accepted in such regions. Comparisons between cultures that have historically positioned their infants supine during sleep (e.g., Japan, Korea, India, and Pakistan) and those that have traditionally practiced prone positioning (e.g., United States, Canada, Nigeria) demonstrate a higher CI in the former populations.
The Real Problem
Deformational flattening occurs only when cranial expansion and growth are consistently resisted in a specific area by an external force. The cranium grows passively in response to minor internal pressure exerted by the rapidly growing infant brain. This process is fastest in early infancy and tapers dramatically even after the first year of life. When an infant is placed on a resting surface, there is a contact force generated between the head and the surface. The force applied by the head to the resting surface equals the weight of the infant’s head multiplied by the force of gravity (F = mg). Newton’s first law predicts that for an object at rest, there will be an equal, but opposite, force from the bed to the infant’s head. This counterforce will resist cranial growth in the area of contact, and consequently, volume increases will be displaced to areas where there is no resistance. Over time, this compensatory growth leads to cranial deformation and flattening. Thus, the pathogenesis of DP, DB, and DS is analogous to how a pumpkin flattens as it grows in a field- it cannot expand into the ground so it grows around the obstruction. This explains why most parents begin to notice head flattening in their infants at an average of 6 to 8 weeks of age since it takes this long for cranial flattening to occur. A larger pumpkin (i.e., one growing faster) exerts a greater downward force on the ground (and the ground on it) than a smaller pumpkin, and consequently, the degree of flattening that occurs over a given time is proportionately greater. This concept may explain the observation that flattening is more common in male infants, as they have larger and slightly faster-growing heads than female
Flattening occurs gradually as the infant cranium grows against a flat, unyielding resting surface. This only happens when the point of contact between the infant head and resting surface (mattress, car seat, etc.) is consistent through time, usually due to some factor that limits independent head mobility (e.g., torticollis)
Predisposing Factors: Why do some infants become flat, while many others do not?
Even if the mechanism of cranial deformation can be explained, it is not so easy to predict why this occurs in only some infants. Parents of an affected infant often ask why their child’s head flattened, whereas other supine-positioned infants in their baby group or family did not. Many authors have struggled with this question. It is useful to discuss some of these well-intentioned, but misleading, ideas. One commonly held belief is that flattening begins in utero and progresses after birth. According to this logic, the congenital flat head would be the most comfortable and geometrically most likely spot on which the infant would lie. Over time, prolonged contact between the same area of the occiput and the sleeping surface leads to progressive flattening. In support of this view, Petisch and coworkers documented localized flattening in13% of otherwise healthy newborns. The measured asymmetry was in the order of several millimeters and may be in the realm of normal. Graham and colleagues found an average 3 mm of asymmetry in normal 6-month-old infants. It is improbable that such a minor geometric disparity would have a prolonged impact on the infant’s ability to change head position. This theory has been called into question by van Vlimmering and colleagues, who found no correlation between cranial asymmetry at birth and subsequent occipital flattening at 7 weeks.
Back sleeping is another easy etiologic scapegoat. Given the acute rise in observed cases since the Back to Sleep Campaign, it has become an almost knee-jerk response to blame back sleeping exclusively for head flattening. Although back sleeping has clearly increased the observed incidence of cranial deformation, it cannot be the only etiologic factor. Deformational flattening also occurs in prone-positioned infants and was well described long before supine sleeping was commonly practiced in the West. Additionally, most infants who are positioned supine do not develop clinically significant occipital flattening. One factor is that brain growth and maturation (and consequently cranial growth) in the occipital/parietal region of the cranium are more pronounced than the frontal area during infancy. Because deformation is a consequence of redirected growth, it is logical that faster-growing regions would be more affected than slower-growing ones. It is reasonable to hypothesize that back sleeping amplifies the effect of certain risk factors for DP, but is not the cause per se. Because the rate of deformation is increased by back sleeping versus prone sleeping, the severity of flattening that develops in a given time is also greater in the former group than the latter group. Consequently, supine-positioned infants are more likely to be identified as flat.
Limited head rotation is the principal risk factor
Although many risk factors have been correlated with the development of deformational flattening, they all coalesce to one underlying theme- cranial flattening occurs in infants who have limited head mobility early in life. Most term infants develop sufficient strength and coordination to support their head against gravity by 3 months of age, and further flattening is unusual after this age. Thus, any intrinsic or extrinsic factors that limits the ability of the infant to change their head position during the first few months of life greatly increase the likelihood of cranial deformation. Table 1 lists many of the risk factors that have been identified, but they all fall within one of the four causes of limited head mobility- congenital muscular torticollis (most common by far), prematurity, developmental delay, and external constraint.
Torticollis/Cervical Imbalance
The most significant condition that limits head rotation is CMT. This is found in up to 70% to 95% of infants with DP. The reported incidence is highly dependent on the experience of and diagnostic criteria used by the observing clinician. Pivar and Scheuerle found that the published rate of CMT in infants with DP ranged from 5% to 67% in 18 treatment centers in Texas. These authors concluded that this inconsistency did not reflect true patient variability but was instead attributable to differences in the training and experience of the treating clinicians. Another factor that may cause under- reported CMT in infants with DP is the strong tendency for the sternocleidomastoid imbalance to improve during the first year of life. By the time many physicians see these patients, the findings are often minor or gone. Congenital muscular torticollis can transiently slow achievement of early motor milestones, and this can further increase the risk of cranial deformation. The earliest manifestation of CMT is the tendency for the infant to maintain a particular head position despite attempts to reposition. We found that nearly all parents observed this in their plagiocephalic infants. Both parents and clinicians often attribute this tendency to different environmental factors (eg, the side on which they feed the baby, the location of the bed in the room, etc). Nevertheless, attempts to alter the environment are almost invariably unsuccessful in altering the preference.
In my opinion, any infant with a preferred head position has a cervical imbalance, or torticollis, until proven otherwise. Failure to recognize head rotational preference in an infant as de facto torticollis has led some to incorrectly deduce that plagiocephaly can lead to torticollis. In many instances, the classic head tilt associated with CMT manifests only when the infant attempts to balance the head weight against gravity at about 3 to 4 months of age. Before this, the neonatal head is almost constantly supported by a resting surface, and the cervical muscles provide only minimal head sup- port. Until there is a true gravity challenge[ to the cervical muscles, head tilt may not occur. Accordingly, we have found that the presence of a head tilt is a less reliable and late physical finding compared with head rotational discrepancy for diagnosing CMT. Interestingly, the head tilt seen in most 5- to 6-month-old infants with CMT is not related to muscle tightness on the side of the tilt, but weakness of the SCM on the opposite side. The tight SCM often stretches out much earlier (typically by 4Y5 months) than the weak SCM muscle strengthens. The presence of unilateral SCM weakness will result in intermittent head tilt, especially when the child is tired or preoccupied. This type of tilt is not the sign of a contracted muscle, which would create a consistent head tilt and a major head rotational disparity. Failure to understand this difference often leads to fruitless attempts to treat the tight muscle with manual stretching or a cervical collar (e.g., TOT, Symmetric Designs, Salt Spring Island, British Columbia, Canada) when, in fact, the correct management is to strengthen the weak contralateral SC
Active, not passive, range of motion is the most reliable test for torticollis in an infant. This can be assessed as early as a few weeks of age. The infant is induced to turn to each side by the parents. I use crumpled paper in the office, but the parent’s voice or a rattle works well. In this patient, head rotation to the left is excellent. This is, of course, the side that he “prefers” when sleeping and is also flattening.
Head rotation to the right is more limited. The infant tried to hold this position, but could not for long. Thus the “preference” is not volitional, but a result of imbalance of the cervical muscles, especially the sternocleidomastoid.
Head rotation discrepancy demonstrated in an older infant. Active head rotation reveals excellent head rotation to the left. Note the chin point is almost at the shoulder, indicating slight weakness or laxity in the left sternocleidomastoid muscle. This will result in a head tilt to the right side when the infant is fatigued or preoccupied.
Head rotation to the right side about 65 degrees, well off of the shoulder; this indicates modest residual tightness of the right sternocleidomastoid muscle.
Prematurity, Developmental Delay, and Extrinsic Restraints
These reasons are far less common as causes of deformational flattening than CMT, yet they are important to discuss. With an understanding that limited or restricted head mobility is the reason deformational flattening occurs in some infants and not others, it is understandable why infants who are premature or developmentally delayed infants have a higher risk of DP and DB, as each of these diagnoses leads to a temporal delay in independent head mobility. In spite of some inaccurate or nefarious reports suggesting that deformational flattening can cause developmental delay, there is no credible evidence for this. It is, however, true that infants with neurocognitive or neuromuscular delays are at higher risk of becoming flat due to slower motor development. It is also important to recognize that some infants will have more than one of these risk factors. For example, multiple-birth infants are often born premature and, due to crowding in utero, one or more can have CMT. Similarly, severely premature infants may require long periods of ventilator support and/or sedation which completely restricts independent head repositioning. In spite of steps taken to reduce the effects of this artificial external restraint on head shape, many premature infants have evidence of cranial deformation.
Prevention and Treatment
Early Identification and Management of the At-risk Infant
Early identification of at-risk infants offers the best opportunity to prevent deformational flattening. The most important fact to know is whether the infant has a head positional preference the earliest manifestation of congenital muscular torticollis (CMT). This simple finding is the key to identifying most vulnerable infants. I recommend that pediatricians inquire about this tendency during the first well-baby visit. Most parents are cognizant of this trait but do not understand the significance. The answer to this one simple question by pediatricians would help identify 90% of at-risk infants- does your infant have a favorite or preferred head position when lying on their back? To confirm one’s suspicion, cervical range of motion can be easily evaluated with the neonate lying supine. The infant should be stimulated to move actively to either side and as passive motion measurements (i.e., the clinician moving the infant’s head manually) will grossly underdiagnose subtle rotational differences. Significant difference in rotation to either side is indicative of cervical imbalance or torticollis. Any infant who has a notable difference in head rotation to one side, has a positional preference, is premature, or has a medical condition that might delay neuromuscular development should be managed proactively. Traditional recommendations for infants who appear at risk for deformational plagiocephaly (DP) or deformational brachycephaly (DB), or have just started to develop a flat spot, include physical therapy to address the cervical muscular contracture and repositioning. Data to support these recommendations are sparse. Nevertheless, a recent study showed that early physical therapy protocol for infants with a positional preference lowered the incidence of severe DP relative to those who had no intervention. The reported reduction was 46% at 6 months of age and 57% at 12 months of age. Because the authors used specific cutoff measurement (oblique diameter difference index, 91%), and not average change in each group, the degree of improvement after this regimen is unknown. Repositioning is possible only in very young and immobile infants (<4 months of age) and, if done consistently, may serve to prevent or limit further deformation. It is extremely challenging for parents to continually monitor and readjust their infant’s head position. This method is extremely difficult to use in older, mobile infants and is generally ineffective at correcting established flattening. In addition, the Food and Drug Administration (FDA) has raised concerns over the safety and effectiveness of positioning devices.
Another method to prevent or treat early plagiocephaly is to alter the shape of the infant’s sleeping surface so that the occiput rests against a concave and not a flat surface. These devices act to redistribute and diffuse the contact pressure between the infant’s head and the resting surface. Consequently, there is little redistribution of volume as the head grows. Alternative sleeping surfaces do not require altering the infant’s head or body positioning, and the infant remains supine at all times. In this sense, alternative resting surfaces are distinctly different than repositioning devices such as wedges. One case-control study compared treatment of early plagiocephaly using a custom-fabricated foam cup to repositioning and physical therapy for the torticollis. At the end of the 2-month treatment period, improvement in the average transcranial difference was significantly different between the groups (P = 0.000): 11.2 to 3.5 mm in the cup group and 9 to 8 mm in the group who received physical therapy and repositioning. The design has been upgraded to include removable liners of increasing size so that parents can adjust the fit as the infant grows. This product, now sold as the PerfectNoggin Infant Mattress, has been used for over 10 years and has documented clinical success in safely promoting occipital symmetry. For more information visit the web site www.theperfectnoggin.com. I currently recommend this orthotic for all infants 4 months or younger who have evidence of torticollis (ie, head preference), are premature, or have early established head flattening.
The PerfectNoggin Infant Mattress (formerly marketed as the PlagioCradle) provides an anatomically-contoured head rest that, unlike other resting surfaces or torso support systems, is upsized as the infant grows. Because the head rests against a contoured surface during the critical first 4 months of life, it is extremely difficult for flattening to develop when used consistently. In addition, the head rest aligns the neck and torso to reduce airway-restricting neck flexion during sleep. Zechariah is the fourth child (of six) in his family to successfully use this same device. His two older brothers were born before the creation of this and have significant residual posterior flattening.
There are many commercial repositioning devices and alternative sleep surfaces on the market that claim to prevent or treat early DP. With the exception of the orthotic described above, none of these have been shown to be effective in a controlled clinical trial. Some manufacturers have sought FDA approval as a means to legitimize their product, but this is an attestation to safety and not effectiveness. These 510(k) type approvals require the manufacturer to demonstrate that their device is substantially equivalent to others that have already been approved. Clinical trials are not necessary for a 510(k) application, and manufacturers are not permitted to make any claims about the effectiveness of their product. Un- fortunately, such restrictions are rarely followed.
Older Infants
By age 3 months, most infants are beginning to develop head control. They become mobile enough to avoid further flattening. Thus, it is rare to see progression of flattening after 4 months of age (in a term child). Most infants with torticollis will have improved to a point where their head tilt may be subtle, intermittent, or completely absent. Even so, a modest but measurable difference in head rotation to either side is typical. Minor rotational differences or intermittent head tilting often reflects a persistent minor weakness in the SCM muscle opposite the tilt, and physical therapy is not indicated. The usefulness of off-the-shelf orthotic devices or repositioning to address cranial flattening after this age is very limited. Most infants can easily move off of wedges and other such devices and rarely stay in the position in which they were placed. The difficult issue is deciding if treatment is warranted. Unfortunately, the literature offers little guidance in this area because of a lack of consensus about how to define and measure DP or DB. Some authors have proposed strict definitions and standardized measurement techniques, but these recommendations have largely gone unheralded. Consequently, the indications for treatment are quite variable, and most clinicians make this decision based on subjective impression, a clinical grading system, or some method of anthropometric measurement.
Methods of Assessing Severity
Subjective impression is inherently inaccurate and strongly influenced by observer bias. When deciding whether to treat an affected infant, it is important to remember that the parents, and not the clinician, will live with the outcome. Not infrequently, I am asked to consult with distraught parents of older children with unresolved DP or DB. They lament that they had sought early care for their child only to be told by a clinician that the baby’s head looked fine and does not require treatment Assured by the professional opinion, they continue to observe the deformity until the child is too old for treatment. Consequently, in my opinion, the only subjective impression worth considering in the treatment decision is that of the parents.
Direct or indirect anthropometry offers some objective standard on which to predicate treatment. Direct measurements can be easily obtained using an anthropometric caliper. Nevertheless, methods of obtaining data and the interpretation of such measurements are quite variable. Deformational brachycephaly and deformational scaphocephaly (DS) are almost always quantified using cephalic index (CI), which is the maximum width of the head divided by the anteroposterior length. This measurement is standard, and normative data are available. Nevertheless, the indications for treatment are still somewhat arbitrary because normal CI varies significantly among different cultures and has increased in North America after the Back to Sleep Campaign. Deformational plagiocephaly is quantified by the degree of asymmetry using either absolute measurements (e.g., transcranial difference; the difference between 2 oblique head measurements) or cranial ratios (e.g., oblique cranial length ratio; ratio of the longer cross-diagonal to the shorter one). We prefer absolute measurements obtained with an anthropometric caliper for assessing DP. The validity and reliability of this technique have been established. Different landmarks have been proposed, and this makes it difficult to compare studies.
Cephalic index- maximum width divided by maximum length (W/L) x 100. Normal pre-1993 was an average of 0.75. Current estimates in back-slept infants is modestly higher (average 0.8-0.85). This reflects the wider variation in head shape in a back-sleeping population.
Approximate landmarks to measure absolute transcranial difference (long oblique-short oblique).
Finger estimation of transcranial difference. Not precise, but a reasonable proxy for most patients.
Head caliper for direct anthropometry.
Regardless of the technique used, consistency among measurements is critical. We use fixed landmarks that have been de- scribed previously. No method of two-dimensional anthropometry can accurately quantify the three-dimensional volume loss that occurs in DP. Furthermore, standardized anthropometry using fixed anatomic landmarks is not accurate for all variations of plagiocephaly. For example, patients with DS typically have flattening of the parietal region but no significant occipital asymmetry. A standard transcranial measurement may fail to detect a difference, even though there is a volume deficit on one side of the cranium. Despite these limitations, anthropometry is an easy and helpful assessment tool. In the absence of an anthropometric caliper, one can easily estimate the degree of flattening. With the infant’s head centered, finger- tips are placed on opposite sides of the occiput in parallel lines posterior to the anterior globe. Typical finger width is 8 to 11 mm, and this can be used to estimate the offset between the sides of the posterior cranium. While this is imprecise, it is a quick and easy method to screen infants.
Some authors prefer to discuss cranial vault asymmetry in terms of cranial ratios. The primary problem with these measurements is that any fixed asymmetry will seem to improve merely because the head becomes larger. Based on these calculated measurements, some researchers have mistakenly concluded that DP naturally gets better, even though the absolute asymmetry in DP does not decrease significantly. This type of relative improvement is relevant only in infants and young children who are typically viewed from the top of their heads. In these patients, increased head size will make a fixed asymmetry look less impressive relative to the total size of the head. A useful analogy is comparing the appearance of a 1-cm indentation on a baseball with the same-size depression on a soccer ball. Of course, the smaller object looks more severely impacted. Nevertheless, cranial ratios are meaningless in adolescents and adults, because the top of their head is rarely visualized by other persons. The cranium of older, taller persons is seen only from a posterior or side view. Thus, the difference in projection between the 2 sides of the occiput will ultimately determine how the asymmetry appears. This difference is best captured by absolute measures such as transcranial difference.
Helmet Therapy
Helmet orthosis is a useful way to improve moderate and severe deformational flattening. I rarely recommend helmets to parents, as the decision to treat flattening should be left to the parents once they are properly educated on what to expect from each alternative. In my practice, I discuss the option of a helmet when the cranial shape meets certain minimum measurement criteria (transcranial difference: >10 mm in DP, CI > 0.90 in DB), is clearly visible from the posterior and side view, and is of concern to the parents. These devices have an excellent track record of safety and effectiveness. Some skeptics are quick to note the lack of type I evidence (randomized controlled studies) to support the effectiveness of this method, although lower levels of evidence do exist. Others have reported some minor relapse with longer follow-up. The first helmets were made and tested in 1979 by Sterling Clarren. His design concept was simple: if the pressure of a rapidly growing brain against a flat surface would flatten the skull, then pressure (relief) over the concave surface should round it back again.
A well-designed molding helmet modeled by my fourth child, Ava. Note that the helmet has a large foam layer to allow gradual contouring and remodeling without the need for multiple helmets. I am not a fan of see-through plastic helmets as they offer less flexibility and often need to be replaced during treatment. In my experience, all but the most severe head shape differences can be sufficiently treated with a single, well made helmet. Beware of orthotic companies who suggest otherwise.
The mechanism by which helmet therapy induces gradual cranial shape change. This is a passive function wherein the helmet holds certain areas that are overgrown and allows disproportionate growth in the flatter areas. The claim of “active”helmet therapy, or treatment that moves or remodels bone segments, is largely marketing hype as undue pressure on the cranium would create progressive skin breakdown. Skin breakdown is occasionally seen on poorly fitted or over-tight helmets.
A common misconception is that the helmet is actively molding or squeezing the cranium and will cause discomfort. Helmets do not function in this manner and act more like a brace to redirect remaining cranial growth toward the flattened areas. When parents ask how much pressure is being applied to the head with these devices, I remind them how little pressure was required to develop the flattening in the first place- the weight of their newborn’s head against an unyielding surface was enough to redirect cranial growth. Therefore, the amount of contact pressure required to correct the shape is negligible and similar to the pressure applied to the head by a well-fitted hat. If compressive force was applied to the head, as some skeptics suggest, the skin would begin to break down within a matter of days. It is analogous to wearing a pair of tight shoes- the skin will become red and irritated in a few hours, begin to blister after a day, and eventually break down if the pressure persists. The same outcome can be expected with a tight or constricting helmet orthotic. This is one reason why the manufacture of these devices is regulated by the FDA, and a skilled orthotist is necessary to monitor the fit. Certain proprietary helmets are touted as active orthotics or bands and are said to apply pressure over bony prominences to provide a better or faster correction of shape. As mentioned above, the scalp (like any soft tissue of the body) does not tolerate sustained pressure well and any device that applies sufficient external pressure to move or push in a bony prominence would soon cause skin breakdown. Thus, the advantages suggested by these manufacturers are more marketing than fact.
Proprietary claims aside, all helmets generally work in the same fashion. To allow the desired changes, the helmet has a foam liner that is selectively cut away in the area where increased growth is desired. The remaining foam limits growth in areas where there is already excessive expansion. The direction of growth is altered, but the overall cranial growth continues normally and there is no restriction of brain expansion. Over time, excellent correction of flattening can be obtained. Helmets are custom made and are tolerated very well by most infants. Sleep patterns are rarely affected. Helmets are effective as long as there is cranial growth remaining, and the rate of correction is proportionate to the rate of growth. Consequently, younger patients will correct much faster than older ones. Children as old as 18 months of age can still have some correction of flattening, but the process may take as long as 6 to 8 months. In contrast, a 4-month-old with a moderate-to-severe asymmetry can often be corrected in 6 to 8 weeks. The effect of age at treatment has been well described. Orthotic correction of DP is generally faster and more effective than that of DB, and this outcome difference has been attributed to slow growth of the cranial base. However, a more plausible explanation is that correction of DB requires growth of the entire occipital region, whereas DP requires expansion of only one half. Hence, it will take considerably more time to correct DB than DP.
Surface laser scan of patient with left posterior deformational plagiocephaly before (left) and after (right) 4 month of helmet therapy. It is imperative to note that any patient’s result will depend on the quality of the helmet design, the age treatment starts (younger is better), duration of treatment, and compliance with wearing the device (-20+ hours/day minimum). Failure to properly control for these variables, coupled with the misguided use of cranial ratios to evaluate improvement, are the major weakness of most studies suggesting that helmets are not effective.
Some reports suggest that helmet therapy may improve facial asymmetry and ear alignment, but this has been refuted by other studies. Fortunately, the face grows steadily well into adolescence. As a result, small difference in facial projection that appears quite noticeable on a small infant will disappear with growth. These early changes are rarely evident in adult, even if there is still significant cranial asymmetry.
No Treatment- Observation
By age 4 months, most developmentally normal infants have achieved head control, and they can sit with assistance. Consequently, many infants with torticollis typically have marked improvement in head rotation and will be unlikely to have further flattening. It has been suggested that once the infant begins to sit up or roll over, the flat spot will spontaneously pop out. This rather absurd notion implies that the cranium can grow differentially, that is, speeding up on the flat side while slowing down of the round side. There is no physiological mechanism by which this can occur. Once the infant can reposition the head, the sustained obstruction to growth is removed, and each side of the occiput will grow at a similar rate. Thus, the degree of flattening remains unchanged. Nevertheless, many observers have stated that the flattening looks less noticeable as the child’s head grows. These seemingly conflicting observations are based on 2 factors. First, the head continues to expand even after there is no further flattening. Thus, relative to overall head size, the flattening appears less pronounced. Compare an indentation on an apple with the same size indentation on a cantaloupe. The flat spot appears less obvious on the larger, wider fruit. It is no coincidence that studies that used either subjective visual assessment or cranial ratios typically reported improvement of DP over time. In contrast, other studies that used absolute differences (transcranial difference) show only minor improvement. Numerous other studies have also documented incomplete correction with growth. Cranial growth is finite and nearly complete in early childhood. Therefore, in severe cases, there may not be enough remaining growth left to normalize appearance. One follow-up study found that 58% of parents still noticed residual asymmetry in their older children (mean age, 7.1 years), although only a small percentage reported being teased by peers. The brain is fully grown by this age, and unfortunately, further improvement through intracranial expansion is impossible.
The second important factor that makes cranial shape look better with growth is the change in an observer’s perspective. Cranial asymmetry is more obvious when viewed from the top of the head than from the back or sides. This is because size difference between 2 objects (including the 2 sides of the occiput) is easier to quantify when they are viewed in a plane perpendicular to the axis in which the discrepancy occurs. For example, if one is looking directly down from an airplane on the top of the Sears Tower, it would be difficult if not impossible to determine the difference in height between it and the adjacent shorter buildings. Nevertheless, the contrast in building height is easy to visualize from the ground. Similarly, cranial asymmetry (which occurs in the sagittal plane of the head) is much more obvious when viewed from the top of the head (axial plane). As a child grows taller, this perspective is almost irrelevant. With the exception of perhaps shoe salesmen, the vertex of an adult’s head is rarely seen by casual observers. In most instances, the cranium will be viewed from the back or the sides, both of which are much more forgiving for those with established asymmetry. In my experience, occipital asymmetry of 10 to 12 mm and greater is obvious in older children and adults with very short hair. As a general rule, if the contrast between the sides of the occiput is easily seen from a posterior or side view in infancy, it is likely that it will be even after growth.
The mitigating effect of perspective. This young man, a family friend, has persistent severe right occipital flattening that affects his ability to wear glasses and helmets. Although the asymmetry is extremely visible on the vertex view, it is almost imperceptible from a posterior view even with short hair.
Hair growth can also provide some camouflage for persistent cranial deformation. It is commonly suggested by parents and pediatricians alike as the remedy for residual flattening. This forces one to adopt a longer hairstyle throughout life to compensate for the asymmetry or flattening. Even with longer hair, the shape of the head can be visualized if the hair is long and straight, is worn on top of the head, or if it is wet. Additionally, hair does little to cover the increased head width observed in children with DB. These children are often referred to by their parents as having a big head, when in fact their overall head size may be normal. In general, hair growth should not be factored into the decision to treat cranial flattening or asymmetry.
Significant residual right occipital flattening in a 12 year-old. The patient’s mother was aware of his head asymmetry, although he was not. Many providers incorrectly counsel parents that the flattening “goes away” with growth.
Instead, flattening that is established at 6-8 months of age often persists but is mitigated by cranial growth, hair coverage, and a shift in perspective from top-down in early childhood to posterior-side in older kids and adult. This patient’s asymmetry is very hard to appreciate when viewed from the posterior perspective.
Although most authorities believe that the only potential long- term effect of deformational flattening is altered cranial shape, some reports have suggested that DP can have medical consequences, such as intellectual impairment or developmental delays visual disturbances, otitis media, decreased motor tone, and occlusal problems. These studies suffer from methodological flaws and have been justifiably criticized. Although infants with develop- mental delay may be more prone to develop DP (presumably due to poor early mobility), few investigators believe that the process of flattening actually causes these delays.In our experience, the overwhelming majority of infants who develop DP or DB have no identifiable developmental delay, and in those who do, there is a clear comorbid condition that accounts for such a finding. The facial asymmetry that is often associated with DP or DS and seems to improve with growth. The face, unlike the cranium, continues to grow well into adolescence. Consequently, facial asymmetries that may be noted in an infant with DP or DS tend to become progressively less obvious over time. The improvement is relative and not absolute, and severe degrees of sagittal (anterior) shifting of auricular position can affect the fit of glasses later in life.