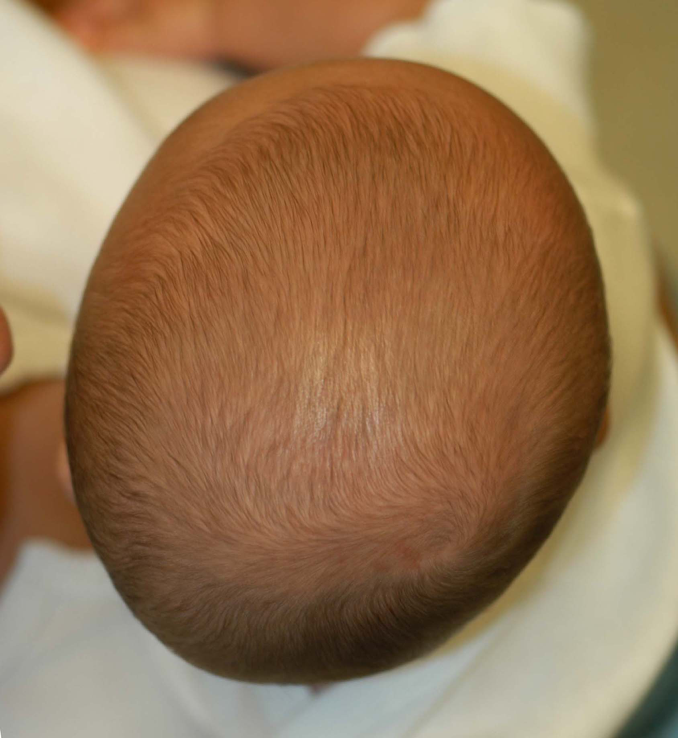
Deformational scaphocephaly (DS) is a less common variant of plagiocephaly seen in infants who have extreme head rotation to one side or in premature infants who are positioned side-to-side in the intensive care units. Flattening develops on the side(s) of the head, with compensatory growth in the anterior and posterior cranium. These infants tend to develop a long, slender head that some refer to as a “toaster head”. There is often relatively pronounced facial asymmetry. This presentation can be confused with synostostotic scaphocephaly associated with sagittal craniosynostosis. Unlike DS, however, sagittal synostosis typically results in frontal bossing, bilateral occipital/parietal narrowing posterior to the anterior fontanelle, and decreased vertical height of the posterior cranium. Facial asymmetry is also rare in sagittal synostosis. Additionally, most infants with this type of craniosynostosis have a head circumferences in excess of the 90th percentile.
The Mechanism of Cranial Flattening: How does an infant’s head become flat?
Several hypotheses have been proposed to explain deformational calvarial flattening. It is often suggested that the infantile cranium is soft or malleable and that this predisposes the bony plates to deform when the head lies on a planar surface. This proposed mechanism is analogous to the type of shape distortion that occurs when a water balloon is placed on a table. This concept is easily dismissed by merely placing a newborn on a firm, flat surface; the head does not immediately deform. Furthermore, because all infants would conceptually have soft heads, one would expect every newborn to undergo cranial flattening if this were true. In reality, less than a quarter of infants positioned on their back develop visible cranial flattening, and the severity peaks around 4 months of age. In an attempt to reconcile these facts, some have proposed that susceptible infants may have an inherent problem with bone mineralization that makes them more susceptible. There is no evidence to support this hypothesis either.
Another common, but generally incorrect, theme is that cranial flattening is hereditary. It is not uncommon for the parents to suggest that some member of the extended family has a similar cranial shape. This is particularly true in families whose cultural traditions include back sleeping. Although ethnic variations in the growth of the cranial base are largely genetic in origin, growth of the neurocranium (calvaria) occurs passively in response to expansion of the brain and intracranial contents. Because the human cerebrum is not naturally flat or asymmetric, flattening can occur only when external force is exerted by a planar surface.
To better understand the mechanism of cranial deformation, it is instructive to look back in history. Intentional cranial deformation is the volitional alteration of normal head shape. This was practiced by many cultures including the ancient Peruvians, the North American Chinook Indians, and the French aristocracy. In most instances, cranial deformation was accomplished by applying a constant external force, usually exerted by a board or cloth wrap, to the growing head. Over time, the natural shape of the head was permanently altered in a predictable and, presumably, culturally desirable way. Although intentional cranial deformation is no longer practiced, some culturally based rearing practices can lead to unintentional changes in cranial shape. For example, swaddle boards (hard, flat infant resting surface) are used in some Asian cultures. Swaddling diminishes infant mobility and, when coupled with supine positioning on a hard, flat resting surface, this results in a high rate of occipital flattening. Not surprisingly, cranial flattening is more common and culturally accepted in such regions. Comparisons between cultures that have historically positioned their infants supine during sleep (e.g., Japan, Korea, India, and Pakistan) and those that have traditionally practiced prone positioning (e.g., United States, Canada, Nigeria) demonstrate a higher CI in the former populations.
The Real Problem
Deformational flattening occurs only when cranial expansion and growth are consistently resisted in a specific area by an external force. The cranium grows passively in response to minor internal pressure exerted by the rapidly growing infant brain. This process is fastest in early infancy and tapers dramatically even after the first year of life. When an infant is placed on a resting surface, there is a contact force generated between the head and the surface. The force applied by the head to the resting surface equals the weight of the infant’s head multiplied by the force of gravity (F = mg). Newton’s first law predicts that for an object at rest, there will be an equal, but opposite, force from the bed to the infant’s head. This counterforce will resist cranial growth in the area of contact, and consequently, volume increases will be displaced to areas where there is no resistance. Over time, this compensatory growth leads to cranial deformation and flattening. Thus, the pathogenesis of DP, DB, and DS is analogous to how a pumpkin flattens as it grows in a field- it cannot expand into the ground so it grows around the obstruction. This explains why most parents begin to notice head flattening in their infants at an average of 6 to 8 weeks of age since it takes this long for cranial flattening to occur. A larger pumpkin (i.e., one growing faster) exerts a greater downward force on the ground (and the ground on it) than a smaller pumpkin, and consequently, the degree of flattening that occurs over a given time is proportionately greater. This concept may explain the observation that flattening is more common in male infants, as they have larger and slightly faster-growing heads than female